There is a lot of variation in bauxites. Structurally they can be solid and dense or crumbly. The usual colour is brick red, flaming red or brown because of iron oxide. If iron content is low, bauxite can be grey or white. But yellow, dark green and even multi-coloured bauxites with bluish, purple, red and black strains occur too.
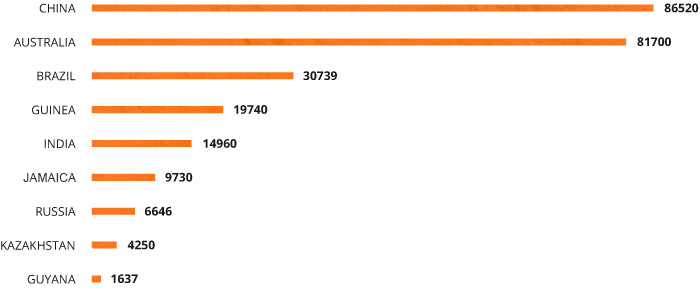
About 90% of global bauxite supplies are found in tropical and subtropical areas, with 73% found in just five countries: Guinea, Brazil, Jamaica, Australia and India. Guinea has the largest supply of bauxites, 5.3 billion tonnes (28.4% of the global supply) and the Guinean bauxites are very high quality, containing minimal amounts of admixtures. They're also found very near to the surface, which makes mining them very easy.